Introduction
Despite improved prognosis with combined antiretroviral therapy (cART) among patients with human immunodeficiency virus (PWHIV) diagnosed with diffuse large B-cell lymphoma (DLBCL), population-based studies suggest inferior survival rates compared to patients without HIV. Uncertainty remains about the persistence of this survival difference across age groups. Examining outcomes across ages may help identify higher-risk groups in the current era of cART, providing insights for designing and conducting age-specific interventions.
Methods
A retrospective population-based cohort was conducted using US population-based cancer registry data among patients aged 15-79 years with newly diagnosed DLBCL from 2010 to 2017, with follow-up through 2019. The 2010-2017 period reflects the availability of integrase inhibitors and the introduction of distinct lymphoma subtypes to the World Health Organization classification system in cancer registries. HIV status was reported at lymphoma diagnosis. Patients were classified into three age groups: adolescents and young adults (AYAs, 15-39 years), adults (40-59 years), and older adults (60-79 years). Overall survival (OS) was defined from diagnosis to death of any cause. Probabilities were estimated using the Kaplan-Meier method and compared with the log-rank test. Multivariable Cox regression models were fitted to assess all-cause mortality, adjusting for cancer stage, race/ethnicity, sex, year of diagnosis, and age. We estimated the cumulative incidence of infections and HIV-related deaths using the competing risk method.
Results
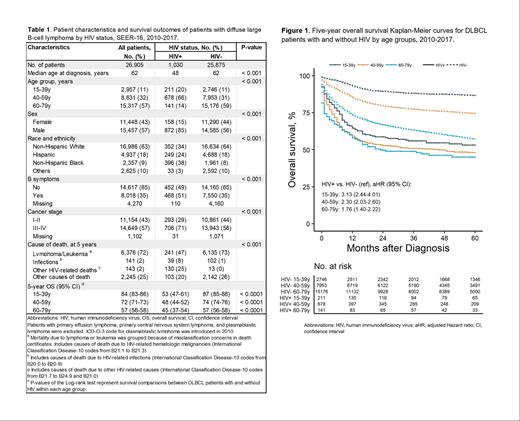
Patient characteristics: A total of 26,905 DLBCL patients were identified (1,030 PWHIV and 25,758 patients without HIV). Compared to patients without HIV, PWHIV were younger (median age at diagnosis 48 years vs. 62 years), predominantly male (85% vs. 56%), more likely to be non-Hispanic Black (38% vs. 8%) and Hispanic (24% vs. 18%), and more likely to have advanced-stage disease at diagnosis (71% vs. 56%) (Table 1).
OS in DLBCL PWHIV vs. patients without HIV:Within each age group, OS was significantly lower among PWHIV with DLBCL compared to patients without HIV(Table 1 and Figure 1). With a median follow-up of 67 months, 5-year OS was particularly low among AYAs (53% vs. 87%, p<0.0001) and adults (48% vs. 74%, p<0.0001). Similarly, older adults experienced lower 5-year OS rates (45% vs. 57%, p<0.0001). Multivariable Cox models suggest that PWHIV has an increased risk of all-cause mortality within each age group (Figure 1).
OS in different age groups by HIV status: For PWHIV, age was not a significant factor for increased all-cause mortality, with 5-year OS rates ranging from 45% in adults to 53% in AYAs (p=0.170). Conversely, in DLBCL patients without HIV, AYAs had superior 5-year OS rates (87%, p<0.001) compared to adults (74%) and older adults (57%).
Cause of death by HIV status: Among both PWHIV and without HIV, lymphoma/leukemia-related mortality was the most common cause of death reported in death certificates (47% and 73% respectively). However, the 5-year cumulative incidence of infection mortality was higher among PWHIV (range 3-4% across age groups) compared to patients without HIV (1% across age groups). For PWHIV, other HIV-related mortality had a 5-year cumulative incidence ranging from 9% to 14% across age groups.
Results stratified by cancer stage (early- or advanced-stage disease) were consistent with those presented above.
Conclusion
This study highlights worse outcomes among PWHIV diagnosed with DLBCL compared to patients without HIV within each age group at the population level in the modern era of cART, notably among AYAs. Unlike the findings in DLBCL patients without HIV, age was not significantly associated with mortality among PWHIV with DLBCL. Future studies should explore age-specific demographic, biological, or healthcare system factors mediating these outcome disparities. The considerable HIV-related mortality suggests refining HIV surveillance approaches among PWHIV with DLBCL in prospective studies or clinical trials. Identifying the optimal integration of chemotherapy and cART may improve outcomes in this vulnerable population.
Disclosures
Castillo:Cellectar: Consultancy, Research Funding; Kite: Consultancy; Pharmacyclics: Consultancy, Research Funding; Loxo: Consultancy, Research Funding; Mustang Bio: Consultancy; Abbvie: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; BeiGene: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal